Story by Adriana Cargill and Lydia Randall
Video by Lydia Randall and Adriana Cargill
Produced by Jin Wu
TAO PUN, Thailand – A black, cloud-like mass billows from a cave on a mountainside in Tao Pun, Thailand. The dark shape rises skyward, shifts, and pivots as the sun sets behind the golden spires of a nearby Buddhist monastery and the green rice fields of western Thailand.
It’s not smoke emerging from the cave, but Thailand’s largest bat colony, embarking on its nightly search for a tasty meal of insects. And just below the mouth of the cave is a team of Thai scientists, waiting to catch some of the bats as they depart.
But the scientists are less interested in the bats themselves than in the viruses they might be carrying.
They’re especially on the lookout for zoonotic diseases — ones that jump from animals to humans. Over the last 60 years, this type of pathogen accounted for more than 70 percent of emerging infectious diseases globally. Ebola, which many believe humans first contracted through contact with fruit bats or primates, has killed thousands. The human immunodeficiency virus, or HIV, a disease that originated in primates, has killed an estimated 34 million people.

Another virus known as Severe Acute Respiratory Syndrome, or SARS, also has been deadly – and economically devastating – since first appearing in late 2002. It too is believed to have originated in bats, including ones similar to those emerging from the cave, which is adjacent to the Khao Chong Phran monastery.
This cave is on the frontline of a war against an enemy humans don’t even know yet, but that might prove disastrous if we miss it. The researchers’ work is done against the backdrop of widespread fear in the public health community that an undiscovered and highly lethal, highly contagious virus might jump from animals to humans.
The scientists plunge thin mesh nets into the air, catching a couple of bats at a time. The winged creatures are small, about the size of a kiwi, and fit easily into their blue latex-gloved palms. They ease the specimens into small cloth bags that begin to jiggle and swing back and forth as the bats fight to escape.
These bat-catching researchers are on the front line of a new global concept, officially launched in 2008, called One Health. It’s a pioneering approach to tackling one of the world’s most difficult challenges: predicting and preventing the next disease that knows no boundaries and could potentially kill millions.
As globalization, air travel and tourism increase, people are pushing further into previously uninhabited areas and into contact with animals that they’ve never encountered before – and, possibly, with new variations of diseases. And with climate change and conflict threatening to displace potentially millions of people and animals, this is a problem that is bound to get worse, and soon.
1. A perfect storm brewing
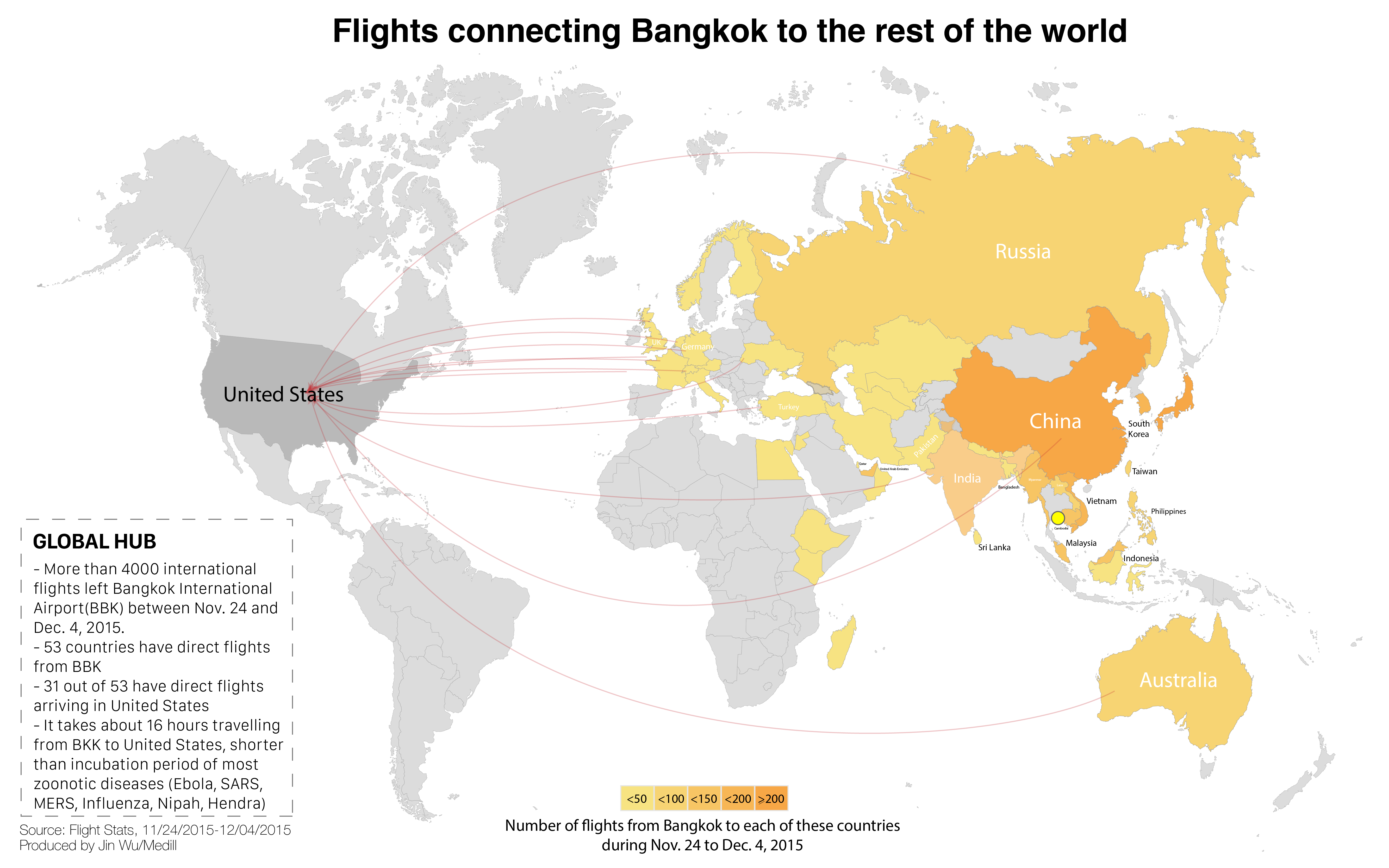
In an increasingly interconnected world, it takes a single virus, hitching a ride on an infected human riding a jetliner, less than 24 hours to reach most of the world’s metropolises, where it can then spread out of control.
Thailand is a hotbed of undiagnosed illnesses and viruses, medical experts say. And Bangkok — its capital, which lies just two hours away from the cave — is a major international transportation hub. That’s why the work of these scientists is so crucial in understanding, and possibly preventing, zoonotic diseases.
“What we are doing is an early detection,” said Dr. Supaporn Wacharapluesadee, who is leading the bat-sampling trip. “We keep an eye on wild animals that could be a potential host to pathogens so that we can figure out in time how to prevent the outbreak.”
Virus Sharing Process

Supaporn, who goes by the nickname Chu, is one of the world’s leading experts on bat pathogens, and the associate director of the World Health Organization’s Collaborating Center for Research and Training on Viral Zoonoses at Chulalongkorn University in Bangkok. Her team was the first in Thailand to study bat pathogens.
Bats are known to be the host for some of history’s nastiest viruses, including rabies, SARS, Hendra and Nipah. And they are likely hosts for new ones, like Middle East Respiratory Syndrome, or MERS, and new strains of Ebola.
The global scientific community has identified the vast majority of the world’s animal species. But scientists don’t fully understand the sheer diversity of viruses that exist within them — or what’s driving viruses to evolve into deadlier and more easily transmittable pathogens.
Many viruses don’t make humans sick, thanks to immunities developed over many millennia. For example, the Ebola virus lives in reservoir hosts that it doesn’t harm, like bats. The problem occurs when viruses ‘spill over’ from bats or other wildlife into humans who lack an immunity to them, with often deadly consequences.
In order to stop viral spillover from happening, scientists like Supaporn are trying to understand how and why it occurs, and the conditions that drive its evolution.

Supaporn’s research on bats has made her a giant in the field of virus discovery worldwide. She’s caught the attention — and funding — of the US government, which funded her research through the US Agency for International Development or USAID and even through the Defense Advanced Research Projects Agency or DARPA, the Department of Defense's research arm.
“I work with some of the global leaders on virologic expeditions and I consider Chu at the very top of my list,” said Dr. Michael Callahan, a US government clinical infectious disease specialist and the founder of DARPA’s Prophecy program, which monitors and predicts virus evolution around the globe in order to prevent and contain outbreaks before they become pandemics.
Without her tireless work ethic and ability to navigate the needs of different governments and the worldwide scientific community, Callahan said, the US government would not have been able to work in Thailand on critically important global virology projects.

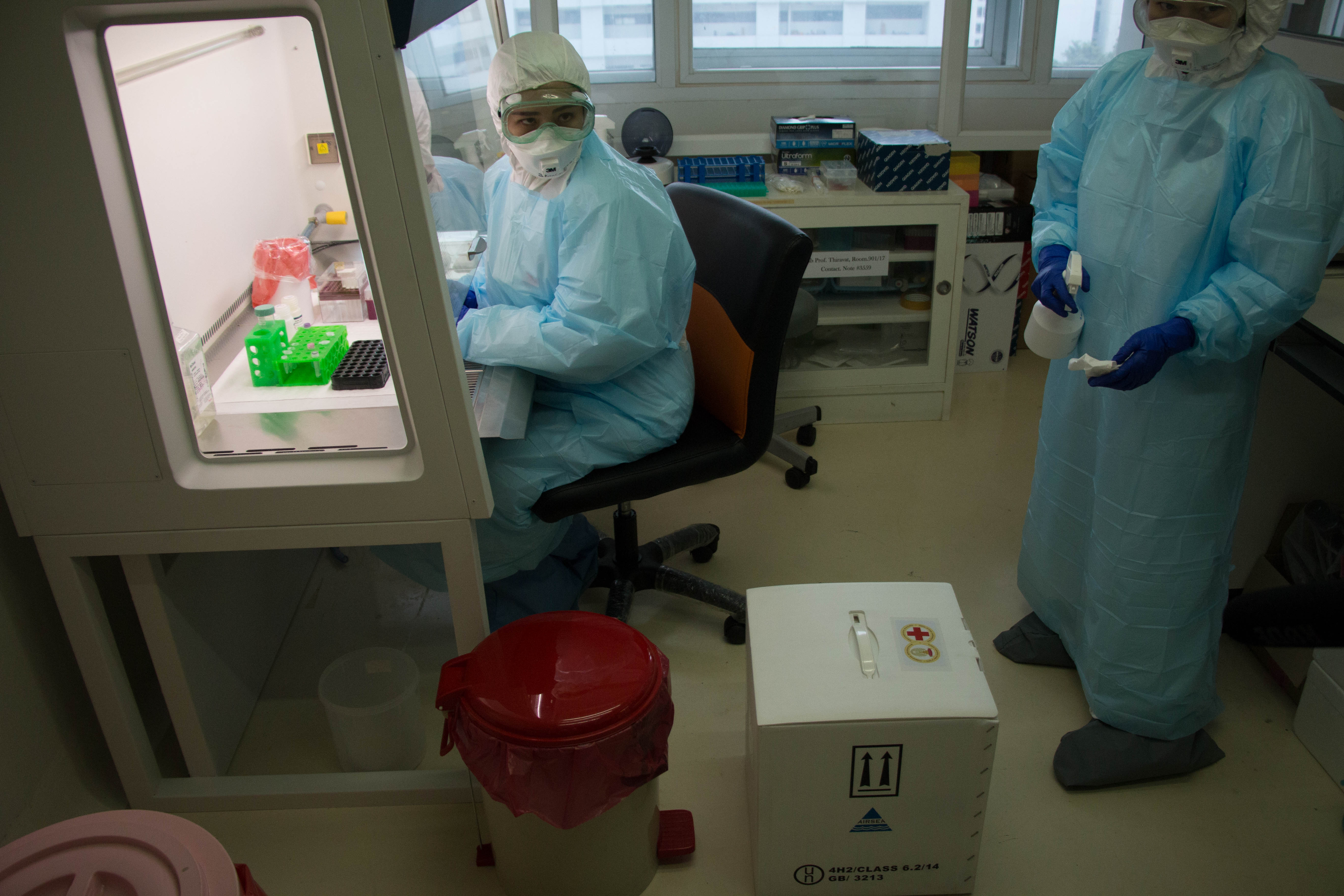
As the bat collectors come down from the mountain, Supaporn and her team of research scientists wait for them at a low-slung concrete Thai Department of National Parks, Wildlife and Plant Conservation building across the street from the monastery. They have turned the outpost into a processing station, where faculty and students from Kasetsart University Wildlife Ecology department help them understand what viruses are coming from which bats.
Students record the type of bat and measure every part of their body. Then they hand them over to Supaporn’s team of research scientists, who are mostly women in their 20s and early 30s. The young virologists from Chulalongkorn University work late into the night taking blood, saliva, urine and feces samples to determine what pathogens the bats carry.
Despite the stifling heat and humidity, they focus intently on the wriggling bats, taking short breaks only to snap a few selfies, and have a quick dinner of sticky rice and sweet pork skewers. Then it’s back to work.
2. Identifying a global threat
The team is up again at 5:30 am, and quickly returns to the cave to capture more bats. After taking more samples, they release them, pack their equipment and pile into a van for the trip home to Bangkok. Then they begin the painstaking lab work to identify any viruses in the bats they captured from the cave.
They will sequence, or genetically fingerprint, the samples using a test called a “PCR assay,” which looks for many viral families at once, especially fast-moving diseases like SARS and MERS.
Unlike Ebola, which requires direct contact with bodily fluids — blood, feces, urine — from an infected animal or person, both SARS and MERS are thought to be spread by respiratory means, like coughing or sneezing, and are therefore much more contagious. Bats are known carriers of both types of viruses.
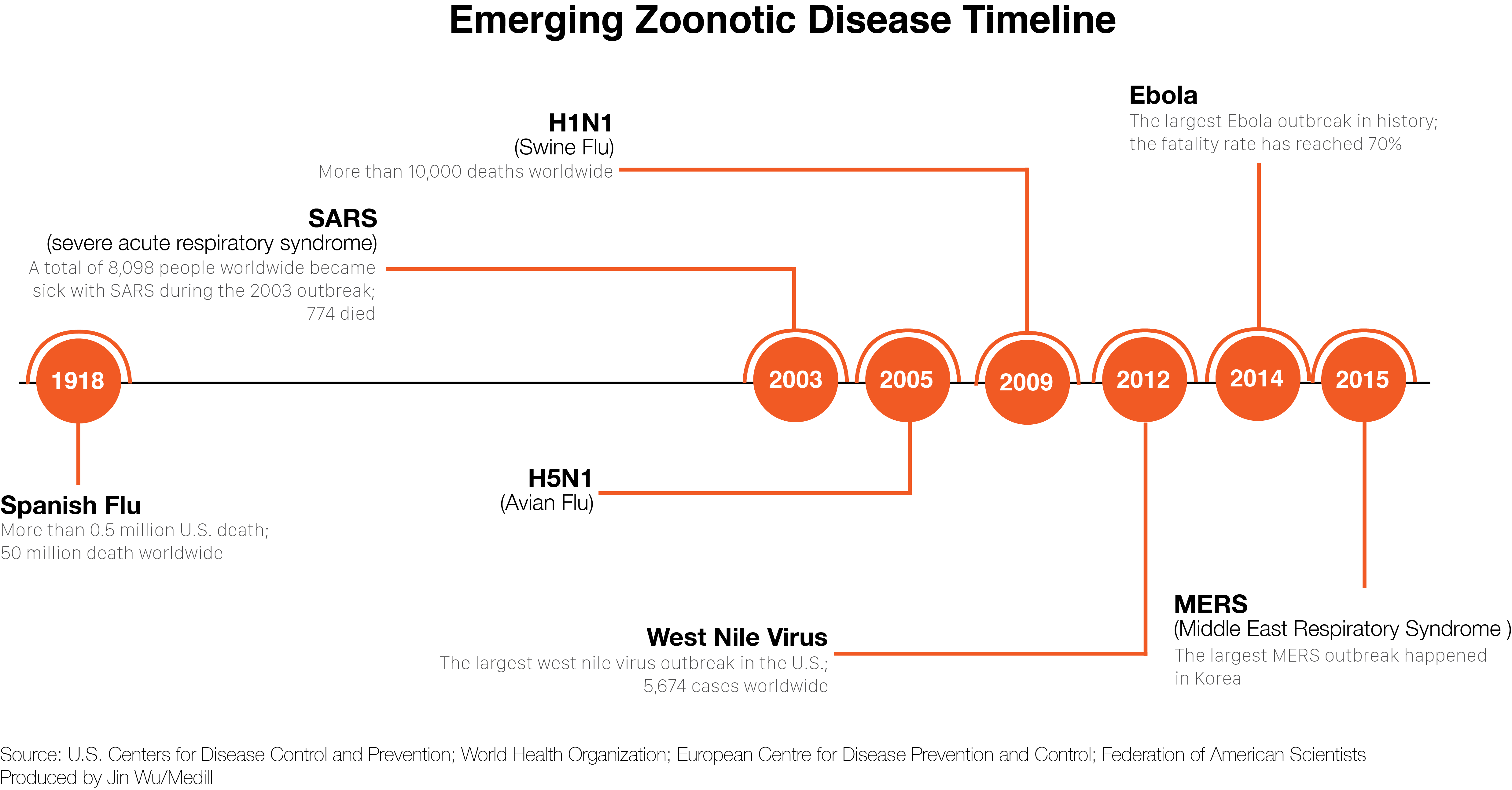
And the emergence of avian influenza, or H5N1, in Southeast Asia in December of 2003 was a reminder of the unpredictability and speed with which viruses can spread. It killed millions of domestic chickens in a matter of months. No virus had ever spread so fast, so far, so quickly. It also began to kill not just birds but leopards, tigers, and even some humans who came into contact with infected chickens.
Influenzas kill at a low rate but can infect a lot of people, which means the numbers can be staggering. The Spanish influenza outbreak of 1918 killed as many as 50 million people, even though it only killed an estimated 1percent to 3 percent of those infected.
In the H5N1 virus, human-to-human transmission had been documented only in people who had prolonged contact with an infected person.
Although it originated in wild birds, the H5N1 virus needed only to make the jump to becoming easily transmitted from human to human for it to become the perfect storm that the public health community had long feared — a virus that was both lethal and highly contagious.
At a news conference in 2005, David Nabarro, the newly appointed Senior United Nations System Coordinator for Avian and Human Influenza, said it was “very likely” that the bird flu virus could jump into the human population. The number of deaths that would result from such a pandemic would depend on where the outbreak occurred, he said, and how quickly and aggressively health officials responded to it. That was especially the case in countries with limited health-care systems, unable to treat vast numbers of sick people and not equipped to transfer information from local to national authorities, he added.
“I’m not, at the moment, at liberty to give you a prediction on numbers,” Nabarro said, “but I just want to stress, that, let’s say, the range of deaths could be anything from 5 to 150 million.”
US officials reacted quickly. Nabarro’s warning never came to fruition. But public health officials realized that outbreak preparedness and response alone wouldn’t stop the next zoonotic disease outbreak.

In Washington, Congress approved in YEAR TK one-time funding for the study and surveillance of H5N1 and other pathogens in wild birds. Its goal: Controlling and containing a potentially lethal new virus at its source, before it could jump to humans.
Luckily that hasn’t happened. Yet.
In Thailand, Supaporn was already working with disease surveillance in wildlife. In 2004, she was the first to discover Nipah virus in Thai bats, a lethal disease that affects both humans and pigs. It was this research that caught the attention of Callahan and DARPA.
In 2009, USAID launched a new five-year program called the Emerging Pandemic Threats program (EPT-1), to expand on its previous avian influenza work.

Within this, the PREDICT program was established to create an early warning system – not for SARS and Ebola, but for new and emerging diseases that officials didn’t yet know about. It established programs in 21 countries that would be run by a consortium of NGOs, non-profits and universities. In Thailand, Supaporn was tapped as PREDICT’s country coordinator.
By the end of PREDICT’s first phase in 2014, partners had discovered over 900 new viruses in bats, rodents, and nonhuman primates around the world. 60 of those were found at the cave next to the Khao Chong Phran monastery.

“Zoonosis is a huge problem taking place not only in Thailand, but worldwide,” Supaporn said. “Everyone should be cooperative and supportive.”
Callahan agrees.
“We need more Dr. Supaporns of the world, who are in country to deploy within hours to the far reaches of those (…) nations, and be able to get and safely isolate viruses and transport them to appropriate sequencing centers for work up and evaluation,” he said.
3.Ground Zero for Emerging Diseases
Bangkok, where Chulalongkorn University is located, is one of the world’s great capitals, and a chaotic assault on the senses. Food vendors jostle for coveted positions in downtown streets, which are a frenzied yet organized bedlam of motorbikes, cars, and pedestrians battling for space.
Bangkok now plays host to nearly 20 million visitors per year; a New Yorker can reach it in 19 hours, a Parisian in 13. But the city and surrounding countryside aren’t just a popular destination for tourists and business travelers. They’ve also become a hub for some potentially catastrophic emerging viruses.
As populations grow around the world — and industry along with them — humans are increasingly encroaching on previously untouched spaces that are home to other animals. Agricultural production and deforestation destroy the habitats of a wide array of wild animals, bringing them into ever closer contact with humans and potentially creating direct pathways for diseases to emerge.
This is known in public health circles as the human-animal interface, and scientists are looking at it as a key driver of potential outbreaks. That’s especially the case in Thailand, where forested areas decreased from over 50 percent of total land area in the early 1960s to 25 percent in 1998. New laws led to some regeneration in the early 2000s, but the problem continues to exist throughout rapidly developing Southeast Asia.
The rodents, monkeys and birds from these previously forested areas also create health risks each time they are bought, sold, processed, and shipped to markets across the region. And then there is the illicit wildlife trade, a growing problem across the region. Scientists have just begun to research how it may contribute to the spread of disease. They believe that bringing wild animals, often from deep in the jungle, into contact with humans and domestic animals can create opportunities for the spread of zoonotic diseases.

There are few places where the risk of this sort of contamination is more apparent than the animal markets of Southeast Asia, including Chatuchak Weekend Market on the outskirts of Bangkok.
Tourists bartering for novelty t-shirts jostle in overcrowded alleys with vendors selling monkeys, parrots, and fighting cocks. Humans, other mammals, and birds all share a small space. Animals from all over the country are brought here, caged, to be sold. That makes for one giant cocktail of potential disease. In the last few years, authorities have cracked down on the illegal wildlife trade, especially in Chatuchak Market. They’ve also mandated rules such as separating birds from mammals in order to prevent a viral spillover.
Thailand is also directly in the flight path for migratory birds and bats, which makes it even more of an epicenter for the transmission of emerging diseases.

It’s this collision of rural regions and urban density, and the movement of animal and human populations, that also make Bangkok the perfect place to study how diseases spread. It is, in Callahan’s words, the “sweet spot for international surveillance.”
For that reason, the US long ago began establishing public health ties with Thailand. In 1959, the .US Armed Forces Research Medical Institute set up shop there. The US Centers for Disease Control and Prevention, or CDC, has been in Thailand since 1980.
Dr. Supaporn’s efforts are the latest chapter in this longstanding relationship. Using the data she collects from bats, her health surveillance lab has established itself as a leader in public health efforts not just in Southeast Asia, but globally. It is not just investigating the viruses of the future — it's helping to protect the public from those that already exist.
Last June, the lab quickly diagnosed a case of MERS in Bangkok and successfully quarantined the person within 72 hours before he could spread the virus to other people. Quick response to a similar case in January helped Thailand avoid an outbreak.
But when a similar case appeared in South Korea in May 2015, it took nine days and visits to several medical facilities before doctors could properly diagnose and quarantine a sick Korean man who had returned home from the Middle East.
Without the rapid response capability of the Bangkok team, the South Korean case turned into an outbreak that killed at least 33 people, infected another 182, put 2,400 under monitoring according to the World Health Organization, and sent economic tremors through the country.
Containing the Threat
Last June a 75-year-old Omani man arrived sick in Bangkok and was promptly admitted to the hospital. He tested negative for a fever at the airport, but Thai doctors couldn’t diagnose his mysterious illness. Immediately, they sent samples to the Thai Ministry of Health, which sent them to two diagnostic labs.
Both labs were unable to identify the unknown pathogen; 48 hours had now passed and the clock was ticking. Then they sent samples to Dr. Supaporn’s lab. Her team applied the same scientific testing method for this man’s samples as they do for bats. They then sequenced the virus and compared it to an online database of bat viruses. This international library is called GenBank, it’s the U.S. National Institutes of Health’s genetic sequence database containing an annotated collection of all publicly available DNA sequences.
Within 15 hours, Supaporn’s lab had identified the unknown pathogen as Middle East Respiratory Syndrome, or MERS. About 72 hours after the sick man landed, he was quarantined and a potentially major outbreak was averted.
The same thing happened in South Korea in May 2015 but it took nine days for doctors at several medical facilities to properly diagnose and quarantine a sick Korean man who had returned home from the Middle East. By then, the 68-year-old had infected medical staff, visitors and other patients. A single case turned into an outbreak that killed 33 people, infected another 182, put 2,400 under monitoring according to the World Health Organization and sent the entire country into a tailspin in just two months.
South Korea has reported another MERS case as recent as October. No further cases of MERS have been reported in Thailand.
This is how studying bat viruses saves human lives, and it’s this type of collaboration that the global health community is trying to replicate. Thailand was able to avoid South Korea’s fate because of the timely and systematic collaboration between virologists, doctors and public health officials across multiple agencies.
Despite the concerns posed by emerging zoonotic diseases in the region, Thailand is now one of the region’s least likely places for a pandemic to begin because of its strong health infrastructure, the One Health collaboration and the world-class work being done by Dr. Supaporn and her team.
This MERS case underscores how the work being done by Dr. Supaporn and her team at Chulalongkorn University is not only vital to Thai national security, but to global health security as well. To stop a pandemic before it starts, one must understand how viruses mutate, how they spillover and – most importantly – how they travel. Understanding the conditions that allow that spillover to happen, and conducting research in that place, is a vital piece in the puzzle.
4. Monks, Miners, Monkeys
It’s 8:am on a Saturday morning and the local guano miners have already been working for hours, hauling plastic sacks of droppings out of the cave from which the bats emerged.
Monks from Khao Chong Phran Monastery stand around and watch, dressed in saffron robes. They allow the miners limited access so they don’t disturb the bats.
After the detection of a potentially novel coronavirus, similar to the MERS virus, at the cave in 2013, DNP officials started telling the monks and miners to be careful when coming into contact with bat feces and urine. There has never been a documented case of bat-to-human transmission, but because the virus is in the same group as MERS, Supaporn is studying it. Scientists are studying similar viruses in China and Africa to see whether any of them could spill over and create an epidemic.
Meanwhile, Thai guano miners often collect guano without masks or gloves, saying that’s how their ancestors did it without getting sick.

Pranorm, a female miner who prefers to use only her first name, has heard that bats carry disease, and that people who hunt and eat them or mine their guano to sell for fertilizer are prime targets for catching a virus. But she can’t stop. “I am afraid but I have to do it,” said the 49-year-old widow, who has a young child to support. “This is my job. This is my income.”
Guano miners and other people who interact regularly with wildlife are of paramount interest to Supaporn. Identifying the risky conditions and human behaviors that give viruses the chance to jump species could help scientists understand more about how and why viruses evolve.

Supaporn has also received Thai funding to study whether locals at Khao Chong Phran have developed an immunity to the viruses that the bats carry.
And the more precisely scientists and health workers around the world can understand viral evolution and the conditions that drive it, the closer they can come to being able to stop the next viral storm before it happens.